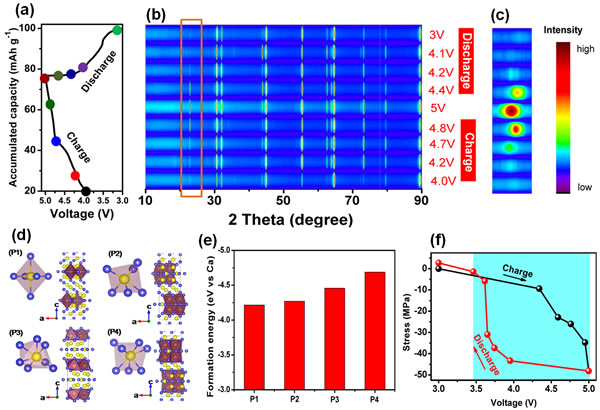
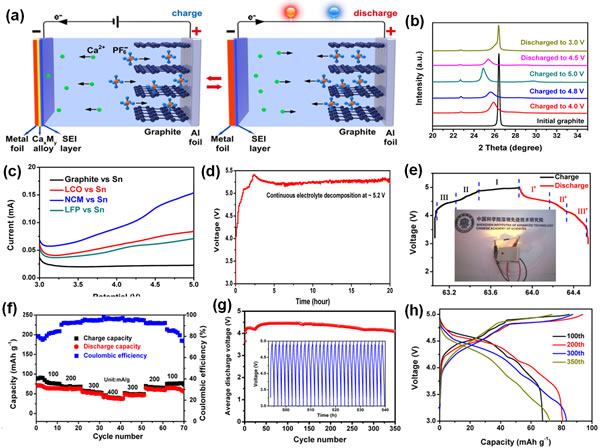
Recently, Tang Yongbing, a researcher at the Functional Film Materials Research Center of the Shenzhen Advanced Technology Research Institute, Chinese Academy of Sciences, and Cheng Huiming, a researcher at the Shenyang Institute of Materials Science, Chinese Academy of Metal Research at the Tsinghua Berkeley Shenzhen Institute and the Institute of Metal Research, Chinese Academy of Sciences, developed a high-performance calcium ion. battery. They through the innovation of the battery structure, so that the calcium ion battery has a new electrochemical reaction mechanism, and to achieve a stable charge-discharge reaction at room temperature. Related research results are published online in Reversible calcium alloying enabling a practical room-temperature charging calcium-ion battery with a high discharge voltage ("High-voltage calcium-ion battery based on calcium-tin alloying reaction and stable operation at room temperature") "Nature" (Nature Chemistry, doi:10.1038/s41557-018-0045-4), a sub-nature of Nature, applied for a Chinese invention patent (201710184368.1) and a PCT patent (PCT/CN2017/078203). Among the alkaline-earth metals, calcium has low polarization, the standard electrode potential is close to that of lithium (Ca2+/Ca:−2.868 V vs. SHE, which is only 170 mV higher than lithium), and the ion is +2 valence (Li-ion number is lithium ion. With twice the advantages of abundant reserves and low cost, calcium ion batteries have the potential to become high-performance and low-cost energy storage batteries. However, in 1991, Aurbach et al. found that in traditional organic electrolytes, calcium ions hardly penetrate the passivation film on the surface of the calcium metal negative electrode, resulting in the inability of calcium ions to undergo a reversible redox reaction like lithium ions (J. Electrochem. Soc. 1991, 138, 3536). Since then, the progress of calcium ion batteries has been slow. Until 2016, MIT's Sadoway et al. used molten CaCl2 and LiCl as electrolytes while using molten Ca-Mg alloy and Bi metal as anode and cathode materials, respectively, to develop a new type of calcium ion liquid battery. Although the voltage is not high (<1V), it exhibits good cycle stability at high temperatures (550-700°C) (Nat. Commun. 2016, 7, 10999). Although Spanish scientist Palacin and others did not find a reversible redox reaction of calcium ions at room temperature, a reversible deposition reaction of calcium ions on the surface of the calcium negative electrode was found in carbonate electrolytes at temperatures between 75-100 °C. It can be circulated for more than 30 weeks at 100°C (Nat. Mater. 2016, 15, 169). Although the discovery of the phenomenon of reversible charge and discharge at high temperatures has brought hope for the development of calcium-ion batteries, but in order to make calcium-ion batteries have practical value, its operating temperature must be reduced to around room temperature, it is necessary to find a way to achieve reversible calcium ion embedding. /Protected positive and negative electrode materials and improve their electrochemical performance, including room temperature cycle characteristics, rate characteristics and operating voltage (currently <2V). After studying the binary phase diagram, the team found that calcium and sodium, zinc, tin and other metals can form alloy phases, and further the charge and discharge characteristics of various metal anodes in carbonate electrolytes containing Ca(PF6)2. A study was conducted and it was found that tin has a good reversible reaction and specific capacity in the calcium ion electrolyte. During the first charging process, the calcium ion in the electrolyte and the tin negative electrode alloyed to form a Ca7Sn6 alloy, and the Ca7Sn6 alloy was decarburized during discharge. Reaction. Theoretical simulations and in-situ electrochemical stress tests show that the four bonding states of calcium and tin in the Ca7Sn6 alloy phase all have lower binding energy, and the electrochemical stress when calcium ions are inserted into the tin negative electrode is compressive stress. The compressive stress not only helps to maintain the structural stability of the material but also has a good reversibility in the process of calcium ion insertion/extraction. Based on the above findings, the team proposed a new type of calcium ion battery: a reversible alloying reaction with calcium ions using tin foil as a negative electrode, and an integrated design of an active material and a current collector; and graphite as a positive electrode to achieve anion (PF6−) ) The reversible intercalation/deintercalation reaction; using a carbonate-type solvent with calcium hexafluorophosphate dissolved therein and 5V high pressure resistance as the electrolyte. The calcium ion battery has excellent electrochemical performance, the average discharge medium pressure is as high as 4.45V, and the capacity retention rate after the cycle of 350 cycles at room temperature is greater than 95%. The work expands the calcium ion battery system, enriches the selection range of key materials such as positive electrode, negative electrode, and electrolyte in the calcium ion battery system, and has important implications for the research and development of novel energy storage devices based on multivalent state ions. Figure 1. (a) The first charge-discharge curve of a tin metal negative electrode in a calcium ion electrolyte; (b,c) XRD analysis shows that an alloying reaction occurs between the calcium ion and the tin negative electrode during the charging process to generate a Ca7Sn6 alloy during discharge. (d,e) Four bond formations and corresponding binding energies of calcium ions and tin in Ca7Sn6 alloy phase; (f) In-situ electrochemical stress tests of tin negative electrode during first charge and discharge curve. Figure 2. (a) Structure and working principle of a novel calcium ion battery; (b) XRD pattern of a graphite cathode at different voltages; (c) Linear scanning of a quaternary pressure-resistant electrolyte system in different anodes on a tin negative battery. Volt-ampere curve; (d) High-voltage resistant test curve of quaternary electrolyte system at a charge current density of 100 mA/g; (e) Charge-discharge curve of a calcium ion battery (a calcium ion button cell can light up two (Yellow LEDs in series), (f) rate performance, (g) change in discharge pressure with number of cycles (inset is a charge-discharge curve of 320-350 cycles), and (h) charge-discharge curve at different cycles . High Transparency Laminated Smart Film
YuGugang have produced standard Intelligent Smart Film for lamination for nearly 8 years. Switchalbe Film used for produced of Laminated Switchable Smart Glass.
PDLC Switchable Smart Film for lamination is available in widths up to 2150mm with a maximum sheet length of 9000mm, which can be cut to any size or shape.
Intelligent Glass already supplies several of the world's largest glass laminators. If your company is looking to produce your own Intelligent Glass, please contact us for more information about becoming an OEM Partner.
A simple ON-OFF Smart Privacy Glass from being clear (transparent) to frosted (opaque). Smart Film its laminated with EVA Film with a standard autoclave.
High Transparency Laminated Smart Film,Laminated Smart Film,Intelligent Film,Smart Film In Building Glass Shenzhen YuGuang New Material Co.,Ltd , https://www.ygsmartfilm.com